Июл . 19, 2024 11:38 Back to list
Basic Steam and Boiler Theory
Basic Steam and Boiler Theory
Basic steam and boiler theory
Steam has been used as a working fluid since the earliest days of the industrial revolution, and it is difficult to appreciate its impact on and importance for the development of that revolution.
Today, its use is common throughout industry not only for mechanical power production, but also for many and varied heating and process applications. The advantages of using steam as the working fluid are partly that it can be easily distributed and controlled, and also that it can serve as the same working fluid for combined power generation and/or heating and/or process work duties.
In addition, water and steam possess the following unusual merits:
- Water is by far the most common liquid on the earth and is therefore plentiful and cheap.
- Water is chemically stable and non-hazardous to health.
- Water is evaporated into steam at temperatures well below the metallurgical limit of boiler steels.
- Water and steam both act as good heat sponges and the evaporation of water into steam also involves a high heat absorption. Hence, plant sizes and costs are not impracticably large.
Steam is the gas phase of the substance with the chemical equation H2O. The solid phase we know as ice, and the liquid phase we know as water. To get steam, we add heat to water so that the water boils, and the water changes phase to steam. When heat is taken away from steam, the steam changes phase back to water.
The change from water to steam, and from steam to water, occurs at a constant temperature. The temperature at which the water boils depends on the pressure on the water – the higher the pressure the higher the boiling temperature. In a boiler the pressure is high and the water boils at about 180°C, which is the same temperature as the steam.
Sensible heat refers to the transfer of heat which can be measured by a change in temperature. The heat transfer can therefore be sensed, hence the name. In our case the transfer of heat to the feed water in the boiler, raising its temperature to the boiling point, is sensible heat.
In order to calculate the amount of heat needed to raise the feed water temperature, we need a property of water called the specific heat capacity, or just specific heat.
The specific heat of a substance is a measure of its capacity to absorb heat. It is the amount of heat that must be supplied to a unit mass of the substance in order to cause a unit increase in temperature of the substance, and its units are therefore kJ/(kg°C) or kJ/(kg K). Its value for a particular substance varies both with its temperature and pressure.
Within the scope of this workshop we are only interested in the specific heat of water. The boilers under consideration here supply saturated steam with no superheat, and thus the specific heat of steam is not relevant. The specific heat of water is of importance as it allows us to calculate the amount of heat needed to raise the temperature of the feed water to the boiling point.
The specific heat of water for our purposes can be taken as 4.19 kJ/(kg°C).
Oil (gas) steam boiler
Latent heat refers to the transfer of heat which cannot be measure by a change in temperature. The boiling process is an example of latent heat transfer. When water boils, its temperature remains constant, and the temperature of the water and the steam are the same. Similarly, when steam gives up heat and condenses back to water, as in a heating coil or drying cylinder, the condensation process also occurs at constant temperature.
Hence, both boiling and condensation are latent heat transfer processes.
The latent heat required to boil water into steam varies with the pressure at which the boiling occurs.
When discussing the pressure of steam in the boiler we must establish early on whether absolute or gauge pressure is being used.
The absolute pressure if the steam is the pressure above absolute zero.
The gauge pressure of the steam is the pressure above atmospheric pressure.
The gauge pressure is always lower than absolute pressure by an amount equal to the atmospheric pressure.
It is common to use gauge pressure when discussing steam, as this is the pressure indicated with standard pressure gauges, but steam tables often use absolute pressure.
Two units of pressure are used when discussing steam. The first is kilopascal (kPa), and the second is bar (b).
There is a simple relationship between the two units: 1 bar = 100 kPa
Temperature is a measure of the degree of hotness or coldness of a substance. The temperature scale commonly used is the Celsius (C) scale, which has a false but convenient zero at the freezing point of water.
The Kelvin scale (K) is also sometimes used which has a true zero at the temperature at which motion ceases. Both scales have the same increments; in other words a temperature difference of 100°C is the same as a temperature difference of 100K.
There is a fixed relationship between the pressure and temperature of saturated steam. This relationship is very useful when it comes to process control, because the process temperature can be set by controlling the steam pressure.
All the thermodynamic properties of steam which are valuable to management are contained in steam tables. The energy values in the steam tables have a zero reference at the freezing point of water. The values are therefore no absolutely accurate, but since we are always concerned with the difference in energy between two points in a system, the absolute values of energy are of no significance.
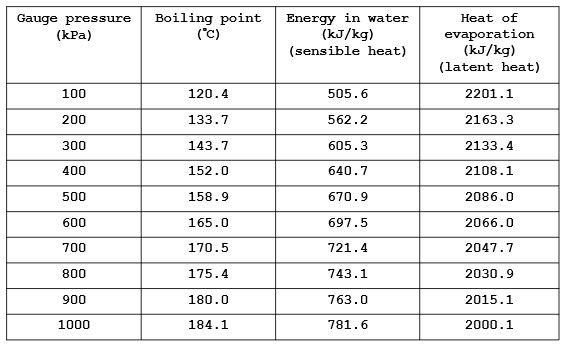
Steam tables may be purchased from technical bookshops, or they may be obtained from steam equipment suppliers. They are also readily available online from a number of website. For ease of reference the following two tables give the energy contained in water and steam which allow for basic steam energy calculations. If the temperature or pressure lies in between the intervals given in the below tables, the straight line interpolation can be used to determine the corresponding values from the tables.

Considering the boiler, probably the most useful energy calculation is that which determines the amount of energy needed to convert feed water to saturated steam.
By way of example, we will consider feed water at 50°C being fed into a boiler which delivers 5 tons of saturated steam per hour at 900 kPa gauge pressure (or 9 bar).
The total energy input is the sensible energy needed to raise the temperature of the feed water to the boiling point, plus the latent heat required to evaporate the water at the boiling point.
Thus, total energy required = (sensible heat of water at boiling point - sensible heat of feed water) + latent heat of evaporation at 900 kPa
Sensible heat of feed water @ 50°C = 209.5 kJ/kg (from Table 2.1)
Boiler point of water at 900 kPa = 180.0°C (from Table 2.2)
Sensible heat of water at boiling point = 763.0 kJ/kg (from Table 2.2)
Latent heat of evaporation at 900 kPa = 2015.1 kJ/kg (from Table 2.2)
So, total heat required = (763.0 - 209.5) + 2015.1 kJ/kg
= 2568.6 kJ/kg
= 2568.6 MJ/ton
The conversion of the energy unit to MJ/ton is useful since we talk of steam quantity in tons, not kilograms.
The actual amount of energy which must be provided to the boiler depends on how efficiently the boiler converts the fuel energy into steam energy, and the above total heat required must be divided by the overall boiler efficiency for the calculation.
A boiler is a machine that continuously produces steam by adding heat to water. The heat typically comes from the combustion of fuel.
The fuel is burned inside the boiler in what is called the fireside. The fireside is a general name given to the spaces inside the boiler where the fuel is burned and where the hot gases from the burning fuel travel through the boiler, and eventually come out from the chimney stack. The steam is produced in the waterside of the boiler, which is the name given to all the spaces inside the boiler where the water is and where the steam is produced.
The two sides, the fireside and the water side, are separated from each other by the walls of the steel tubes and the furnace tube and return chamber. Generally the fireside is the inside of the tubes, and the water side is the outside of the tubes. The heat from the combustion of the fuel travels from the inside of the tube, through the wall of the tube and into the water on the outside of the tube.
Under normal circumstances the boiler controls itself automatically. The fuel (which could be solid, liquid or gas) is burned inside the boiler furnace tube. The amount of fuel burned requires more air than can be obtained from natural movement, and so fans are used to provide the required air. The boiler automatically controls the amount of fuel and air fed into the boiler, and this controls the amount of steam produced. The amount of steam produced should match the amount of steam required by the process.
Because the boiler operates under high pressure, the water has to pumped into the boiler using feed pumps. The boiler automatically controls the operation of the feed pumps to maintain a constant level of water in the boiler. The boiler has various devices to prevent the steam pressure rising to a dangerous level, and also to sound alarms or even shut the boiler down if the water level drops too low.
-
How to Maintain a Steam Boiler Expert Tips for Efficiency & Longevity
NewsApr.29,2025
-
Professional Steam Boiler Service AB Expert Maintenance & Repair
NewsApr.29,2025
-
Hot Water Steam Boilers Efficient Heating Solutions & Expert Tips
NewsApr.29,2025
-
Hot Water Boiler Capacity Calculation Guide Efficient Design Tips
NewsApr.28,2025
-
How to Drain a Steam Boiler Step-by-Step Safety Guide
NewsApr.28,2025
-
How to Install a Hot Water Boiler Optimal Pressure & Efficiency Guide
NewsApr.28,2025
Related PRODUCTS